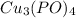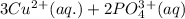 ⇒
⇒
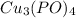
Step-by-step explanation:
Reaction Type
It is double displacement reaction.
Cupric Chloride + Tribasic Potassium Phosphate ⇒ Cupric Phosphate + Potassium Chloride
Reactants
- Cupric Chloride
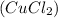
- Tribasic Potassium Phosphate
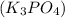
Products
- Cupric Phosphate
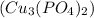
- Potassium Chloride
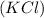
The reaction is as follows -
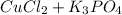 =
=
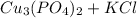
∴ The Balanced Chemical Equation is as follows -
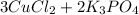 =
=
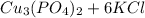
The Net ionic equation including the phases is as follows -
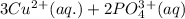 ⇒
⇒