Question:
For a certain commercial jet to be able to achieve liftoff, its total weight cannot exceed 333,390 kg. If the empty weight (i.e., no fuel or passengers) of the jet is 163,293 kg and there are 300 passengers aboard with an average weight of 75.0 kg, determine how many gallons of jet fuel the jet can hold and still be able to takeoff from sea level with an outside temperature of 277 K. The specific gravity of jet fuel is 0.804
Answer:
The answer to the question is;
The jet can hold 48642.2 gal or 40503 galUK of jet fuel and still be able to takeoff from sea level.
Step-by-step explanation:
To solve the question, we note the following
Maximum total weight at liftoff = 333,390 kg
Mass of jet when empty = 163,293 kg
Average mass of one passenger = 75.0 kg
Number of persengers = 300
Total mass of persengers = 300×75 =22500 kg
Mass of jet + passengers = 163293 + 22500 = 185793 kg
Allowable mass jet carry = Mass of jet fuel
= Maximum total weight at liftoff - Mass of jet + passengers
= 333,390 kg - 185,793 kg = 147597 kg
Specific gravity of jet fuel = 0.804 therefore density of jet fuel is given by
Density of jet fuel = Density of water × The specific gravity of jet fuel
= 997 kg/m³ × 0.804 = 801.588 kg/m³
Volume of jet fuel = (Mass /Density) of the jet fuel =
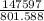 = 184.131 m³
= 184.131 m³
However 1 m³ = 264.172 gal = 219.969 galUK
∴ 184.131 m³ = 264.172× 184.131 gal
= 48642.2 gal = 40503 galUK