Answer:
45000 K .
Step-by-step explanation:
Given :
A liter of a gas weigh 2 gram at 300 kelvin temperature and 1 atm pressure
We need to find the temperature in which 1 litre of the same gas weigh 1 gram
in pressure 75 atm.
We know, by ideal gas equation :

Here , n is no of moles ,
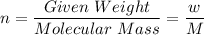
Putting initial and final values and dividing them :
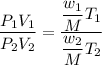
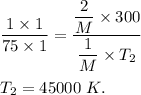
Hence , this is the required solution.