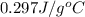Answer : The specific heat of the metal is,
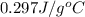
Explanation :
In this problem we assumed that heat given by the hot body is equal to the heat taken by the cold body.
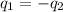
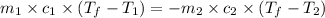
where,
 = specific heat of metal = ?
= specific heat of metal = ?
 = specific heat of water =
= specific heat of water =
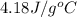
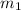 = mass of metal = 75 g
= mass of metal = 75 g
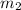 = mass of water =
= mass of water =
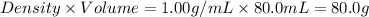
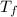 = final temperature of mixture =
= final temperature of mixture =
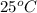
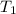 = initial temperature of metal =
= initial temperature of metal =
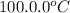
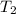 = initial temperature of water =
= initial temperature of water =
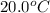
Now put all the given values in the above formula, we get
![(75g)* c_1* (25-100.0)^oC=-[(80.0g)* 4.18J/g^oC* (25-20.0)^oC]](https://img.qammunity.org/2021/formulas/chemistry/high-school/8woucwpftyvz0bzxz15s8rg7lg6fmgj5g0.png)
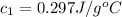
Therefore, the specific heat of the metal is,