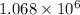Answer: The equilibrium constant for this reaction is
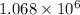
Step-by-step explanation:
The equation used to calculate standard Gibbs free change is of a reaction is:
![\Delta G^o_(rxn)=\sum [n* \Delta G^o_((product))]-\sum [n* \Delta G^o_((reactant))]](https://img.qammunity.org/2021/formulas/chemistry/college/v3nuxeiezeucwhxnsv41ehrhsg3u8mhwjd.png)
For the given chemical reaction:
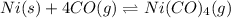
The equation for the standard Gibbs free change of the above reaction is:
![\Delta G^o_(rxn)=[(1* \Delta G^o_((Ni(CO)_4(g))))]-[(1* \Delta G^o_((Ni(s))))+(4* \Delta G^o_((CO(g))))]](https://img.qammunity.org/2021/formulas/chemistry/college/c385vcei8l7pgzow8ecj4sewh4v96gekes.png)
We are given:
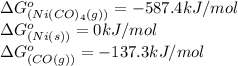
Putting values in above equation, we get:
![\Delta G^o_(rxn)=[(1* (-587.4))]-[(1* (0))+(4* (-137.3))]\\\\\Delta G^o_(rxn)=-38.2kJ/mol](https://img.qammunity.org/2021/formulas/chemistry/college/f78ga011degtq5q7d9yfp1z8vqgprfk6de.png)
To calculate the equilibrium constant (at 58°C) for given value of Gibbs free energy, we use the relation:
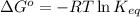
where,
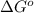 = Standard Gibbs free energy = -38.2 kJ/mol = -38200 J/mol (Conversion factor: 1 kJ = 1000 J )
= Standard Gibbs free energy = -38.2 kJ/mol = -38200 J/mol (Conversion factor: 1 kJ = 1000 J )
R = Gas constant = 8.314 J/K mol
T = temperature =
![58^oC=[273+58]K=331K](https://img.qammunity.org/2021/formulas/chemistry/college/4r4hgl159sfdzaxvu3colkp5jgg1jjfwqj.png)
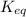 = equilibrium constant at 58°C = ?
= equilibrium constant at 58°C = ?
Putting values in above equation, we get:
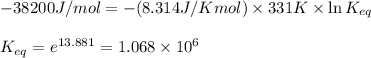
Hence, the equilibrium constant for this reaction is