Answer:
work done in the process is 0 J.
Step-by-step explanation:
Given :
Initial pressure of system ,

Final pressure of system ,

Volume of system ,

We know, In thermodynamics work done is defined as :
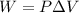
Here , P is pressure and
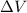 is change in volume.
is change in volume.
Now, coming back to question , it state that the process is isochoric .
Therefore, change in volume ,

Putting value of
 in above equation we get ,
in above equation we get ,
W = 0 J.
Therefore , work done in this process is 0 J.
Hence, this is the required solution.