Answer: The net chemical equation is
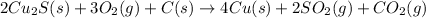
Step-by-step explanation:
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on product side.
Every balanced chemical equation follows law of conservation of mass.
The balanced chemical equation for Step 1 process of the reaction:
Step 1:
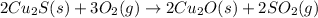
The balanced chemical equation for Step 2 process of the reaction:
Step 2:
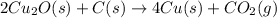
The net chemical equation for the formation of copper from copper(I) sulfide, oxygen and carbon follows:
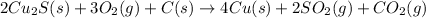
Hence, the net chemical equation is written above.