Answer :
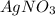 act as oxidizing agent and Cu act as an reducing agent.
act as oxidizing agent and Cu act as an reducing agent.
Explanation :
Redox reaction or Oxidation-reduction reaction : It is defined as the reaction in which the oxidation and reduction reaction takes place simultaneously.
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
Reducing agent : It is defined as the agent which helps the other substance to reduce and itself gets oxidized. Thus, it will undergo oxidation reaction.
Oxidizing agent : It is defined as the agent which helps the other substance to oxidize and itself gets reduced. Thus, it will undergo reduction reaction.
The given chemical reaction is,
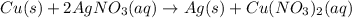
In this reaction, Cu shows oxidation reaction because oxidation state changes from (0) to (+2) and Ag shows reduction reaction because oxidation state changes from (+2) to (0).
The oxidation-reduction half reaction will be :
Oxidation :
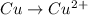
Reduction :
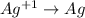
In this reaction,
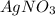 act as oxidizing agent and Cu act as an reducing agent.
act as oxidizing agent and Cu act as an reducing agent.