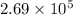Answer: The value of
 for the net reaction is
for the net reaction is
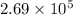
Step-by-step explanation:
The given chemical equations follows:
Equation 1:
![PCl_5(g)\xrightarrow[]{K_1} (1)/(4)P_4(g)+(5)/(2)Cl_2(g)](https://img.qammunity.org/2021/formulas/chemistry/high-school/nifa4mh8wuxwblx3knmqkjv3cp09dwfknc.png)
Equation 2:
![(1)/(4)P_4(g)+(3)/(2)Cl_2(g)\xrightarrow[]{K_2} PCl_5(g)](https://img.qammunity.org/2021/formulas/chemistry/high-school/sdk5e8og3ukwbzdhn0ww9e1i6p66dhw8m3.png)
The net equation follows:
![PCl_5(g)\xrightarrow[]{K_c} PCl_3(g)+Cl_2(g)](https://img.qammunity.org/2021/formulas/chemistry/high-school/1ui4f7mkxvc8m440qrh9o36rwa0e2vjuxm.png)
As, the net reaction is the result of the addition of two above equations. So, the equilibrium constant for the net reaction will be the multiplication of two above equations.
The value of equilibrium constant for net reaction is:
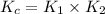
We are given:
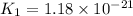
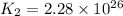
Putting values in above equation, we get:
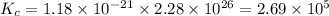
Hence, the value of
 for the net reaction is
for the net reaction is