Answer: 0.52 L of 15 M
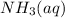 will be used to prepare this amount of 0.52 M base.
will be used to prepare this amount of 0.52 M base.
Step-by-step explanation:
But on diluting the number of moles remain same and thus we can use molarity equation.
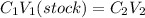 (to be prepared)
(to be prepared)
where,
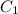 =concentration of stock solution = 15 M
=concentration of stock solution = 15 M
 = volume of stock solution = ?
= volume of stock solution = ?
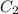 = concentration of solution to be prepared = 0.52 M
= concentration of solution to be prepared = 0.52 M
 = volume of solution to be prepared = 15 L
= volume of solution to be prepared = 15 L
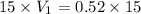
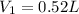
Thus 0.52 L of 15 M
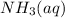 will be used to prepare this amount of 0.52 M base
will be used to prepare this amount of 0.52 M base