The question is incomplete, here is the complete question:
Methane gas and chlorine gas react to form hydrogen chloride gas and carbon tetrachloride gas. What volume of hydrogen chloride would be produced by this reaction if 1.1 mL of methane were consumed? Be sure your answer has the correct number of significant digits.
Answer: The volume of hydrogen chloride produced in the reaction will be 4.4 mL
Step-by-step explanation:
We are given:
Volume of methane gas = 1.1 mL
The chemical equation for the reaction of methane gas and chlorine gas follows:
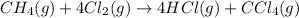
Moles of methane gas = 1 mole
Moles of hydrogen chloride gas = 4 moles
The relationship of number of moles and volume at constant temperature and pressure was given by Avogadro's law. This law states that volume is directly proportional to number of moles at constant temperature and pressure.
The equation used to calculate number of moles is given by:
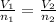
where,
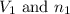 are the volume and number of moles of methane gas
are the volume and number of moles of methane gas
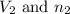 are the volume and number of moles of hydrogen chloride
are the volume and number of moles of hydrogen chloride
We are given:
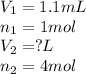
Putting values in above equation, we get:
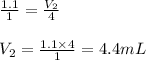
Hence, the volume of hydrogen chloride produced in the reaction will be 4.4 mL