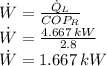Answer:
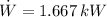
Step-by-step explanation:
A well-sealed house means that there is no mass interaction between air indoors and outdoors. Hence, cooling process is isochoric. The heat removed by the air conditioner is:
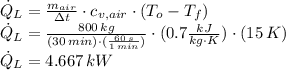
The power drawn by the air conditioner is: