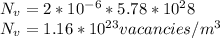Answer:
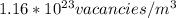
Step-by-step explanation:
Data given
temperature =700c
Density=10.35g/cm^3
but
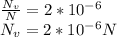
Nv is the number of vacant site and N is the number of lattice site.
Since the number of lattice site can also b computed as
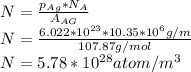
if we substitute the value of the number of lattice into the first equation, we arrive at