Answer:
the mass of CaO present at equilibrium is, 0.01652g
Step-by-step explanation:
![K_p = [CO_2]](https://img.qammunity.org/2021/formulas/chemistry/high-school/njhzaitceiaila4lgghqb7z89oegvr8bje.png) = 3.8×10⁻²
= 3.8×10⁻²
Now we have to calculate the moles of CO₂
Using ideal gas equation,
PV =nRT
P = pressure of gas = 3.8×10⁻²
T = temperature of gas = 1000 K
V = volume of gas = 0.638 L
n = number of moles of gas = ?
R = gas constant = 0.0821 L.atm/mole.k
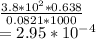
Now we have to calculate the mass of CaO
mass = 2.95 * 10 ⁻⁴ × 56
= 0.01652g
Therefore,
the mass of CaO present at equilibrium is, 0.01652g