Answer:
 acts as oxidizing agent.
acts as oxidizing agent.
Step-by-step explanation:
Oxidation reaction : When there is an increase in oxidation state number.
Reduction reaction : when there is a decrease in oxidation state number.
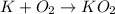
Potassium metal has undergone oxidation, as its oxidation state is changing from 0 to +1. Potassium metal is getting converted into potassium ions.
Oxygen
 has undergone reduction, as its oxidation state is changing from 0 to -1.
has undergone reduction, as its oxidation state is changing from 0 to -1.
The chemical agent which itself get oxidized and reduce others is called reducing agent and the chemical agent which itself get reduced and oxidizes others is called oxidising agent. Thus
 acts as oxidizing agent.
acts as oxidizing agent.