Answer: B. endergonic, not spontaneous
Step-by-step explanation:
Endergonic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants. The total energy is absorbed in the form of heat and
 for the reaction comes out to be positive.
for the reaction comes out to be positive.
The Gibbs equation is:
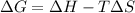
 = Gibb's free energy change
= Gibb's free energy change
 = enthalpy change
= enthalpy change
T = temperature
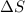 = entropy change
= entropy change
A reaction is non spontaneous when
 = Gibb's free energy change is positive
= Gibb's free energy change is positive
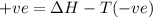

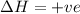
Thus the reaction has to be endergonic.