Answer:
The pH in 0.140 M hippuric acid solution is 2.2.
Explanation :
Dissociation constant of the acid =

![pK_a=-\log[K_a]](https://img.qammunity.org/2021/formulas/chemistry/college/ir8v80n39inyylpe73u5rdi9un2quhm56e.png)
![3.62=-\log[K_a]](https://img.qammunity.org/2021/formulas/chemistry/college/36jlstbc59iz4a7gifniul75hx7p2jsmcf.png)
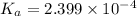
Concentration of hippuric acid = c = 0.140 M
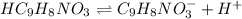
Initially
c 0 0
At equilibrium
(c-x) x x
Concentration of acid = c
![[HC_9H_8NO_3]=0.140 M](https://img.qammunity.org/2021/formulas/chemistry/college/nqczdhgznk8831fmrfweex01h92fahence.png)
Dissociation constant of an acid is given by:
![K_a=([C_9H_8NO_(3)^-][H^+])/([HC_9H_8NO_(3)])](https://img.qammunity.org/2021/formulas/chemistry/college/zd9s4tdo0gpzj9e8icj8s0q11mlfzbek1o.png)
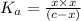
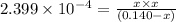
Solving for x:
x = 0.005677 M
![[H^+]=x = 0.005677 M](https://img.qammunity.org/2021/formulas/chemistry/college/ivokcwmv400vmtk14xudmvscn9xge49gm4.png)
The pH of the solution :
![pH=-\log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/fi7xbn2q6p6sosuqayohrecmxrbau6j4s5.png)
![pH=-\log[0.005677 M]=2.246\approx 2.2](https://img.qammunity.org/2021/formulas/chemistry/college/1uo57dignx2v44jmhpbx2h764ql5ljgtb2.png)
The pH in 0.140 M hippuric acid solution is 2.2.