Answer:
0.07789 M is the sodium hydroxide concentration.
Step-by-step explanation:
Mass of potassium hydrogen phthalate = 0.6986 g
Molar mass of potassium hydrogen phthalate = 204.22 g/mol
Moles of potassium hydrogen phthalate =
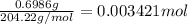
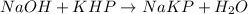
According to reaction , 1 mole og KHp reactswith 1 mole of NaOH , then 0.003421 moles of KHp will react with :
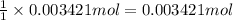
Moles of NaOH = 0.003421 mole
Volume of NaOH solution = 43.92 ml = 0.04392 L ( 1 mL = 0.001L)
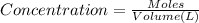
Concentration of NaOH :
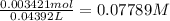
0.07789 M is the sodium hydroxide concentration.