Answer:
The molar mass of the gas is 44.19 g/mol
Step-by-step explanation:
Amount of sample of gas = m = 13.5 g
Volume occupied by the gas = V = 5.10 L
Pressure of the gas = P = 149.83 KPa
1 KPa = 0.00986 atm
P =
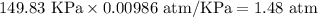
Assuming M g/mol to be the molar mass of the gas
Assuming the gas is behaving as an ideal gas
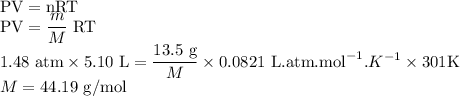
The molar mass of gas is 44.19 g/mol