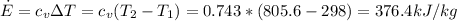Answer:
376.4 kJ/kg
Step-by-step explanation:
T = 25 C = 25 + 273 = 298 K
Since this is an isochoric process, the volume stays constant. The heat transfer comes mainly from internal energy, or change in term of change in temperature.
Assume idea gas law, we have
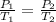
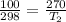
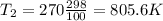
So the heat transfer due to change of temperature is