Answer:
Half-life of the reaction is 0.072s.
Step-by-step explanation:
In a first-order reaction, half-life, t1/2, is defined as:
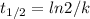 (1)
(1)
Where k is the rate constant of the reaction.
In the problem, the thermally descomposition of cyclopropane has a rate constant of 9.6s⁻¹. Replacing in (1):
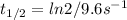
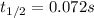
I hope it helps!