Answer:
%
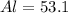 %
%
%
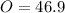 %
%
Step-by-step explanation:
If we know the grams of a chemical compound in a specific reaction, it is possible to know the percentage of each atom that composes it.
For the Aluminum Oxide in this problem, we know its total weight and the grams of each component.
therefore we can determine the percentage ratio of its components through:
For Al
%
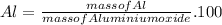 %
%
%
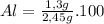 %
%
%
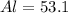 %
%
In the same way for oxygen
%
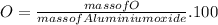 %
%
%
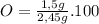 %
%
%
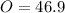 %
%