Step-by-step explanation:
Formula to calculate osmotic pressure is as follows.
Osmotic pressure = concentration × gas constant × temperature( in K)
Temperature =
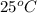
= (25 + 273) K
= 298.15 K
Osmotic pressure = 531 mm Hg or 0.698 atm (as 1 mm Hg = 0.00131)
Putting the given values into the above formula as follows.
0.698 =
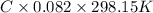
C = 0.0285
This also means that,
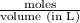 = 0.0285
= 0.0285
So, moles = 0.0285 × volume (in L)
= 0.0285 × 0.100
=
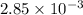
Now, let us assume that mass of
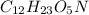 = x grams
= x grams
And, mass of
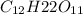 = (1.00 - x)
= (1.00 - x)
So, moles of
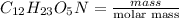
=
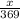
Now, moles of
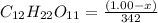
=
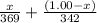
=
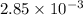
= x = 0.346
Therefore, we can conclude that amount of
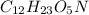 present is 0.346 g and amount of
present is 0.346 g and amount of
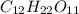 present is (1 - 0.346) g = 0.654 g.
present is (1 - 0.346) g = 0.654 g.