Answer: 60 ml of the 65% mixture will you need to add to obtain the desired solution.
Step-by-step explanation:
According to the dilution law,
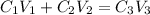
where,
= concentration of given alcohol solution = 25 %
 = volume of given alcohol solution = 100 ml
= volume of given alcohol solution = 100 ml
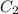 = concentration of another alcohol solution=65 %
= concentration of another alcohol solution=65 %
 = volume of another acid solution= x ml
= volume of another acid solution= x ml
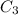 = concentration of resulting alcohol solution = 40 %
= concentration of resulting alcohol solution = 40 %
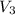 = volume of resulting acid solution = (100+x)
= volume of resulting acid solution = (100+x)
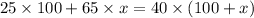
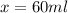
Thus 60 ml of the 65% mixture will you need to add to obtain the desired solution.