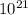Answer:
Number of molecules of H2=4.5454375x
Explanation
Given:
P1=P2=0.5 atmosphere=0.505x
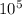 pa
pa
T1=T2=300k
V1=10 liters of O2
N=5.0x
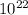 molecules
molecules
10 liters of H2
V2=? liters of O2
H2=1/16 of O2
Procedure:
for every liter of H2, there are 16 liters of O2 since H2=1/16 of O2,
Therefore, 10 liters of H2= 160 liters of O2 i.e.
V2=160 liters of O2.
Applying ideal gas equation,
VP=NkT :where P is pressure, V is volume, N is number of molecules, T is temperature in kelvin and k is Boltzman constant=1.38X
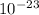 J/K.
J/K.
considering change in volume of O2, N can be calculated as follows:
V1P1=N1kT1..................................(1)
V2P2=N2kT2...............................(2)
dividing (2) by (1)
V2P2/V1P1=N2kT2/N1kT1
but P1=P2 and T1=T2 since they remain the same, they cancel out.
∵ V2/V1=N2/N1
substituting values, we have
160/110=N2/5.0x
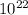
N2=1.4545x5.0x
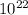 =7.2727x
=7.2727x
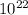 molecules of O2
molecules of O2
Recall that
N2 of H2=1/16 of N2 of O2
∵Number of molecules of H2=1/16 of 7.2727x
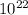
=4.5454x
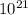
Number of molecules of H2=4.5454375x