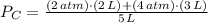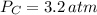Answer:
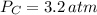
Step-by-step explanation:
Let assume that gases inside bulbs behave as an ideal gas and have the same temperature. Then, conditions of gases before and after valve opened are now modelled:
Bulb A (2 L, 2 atm) - Before opening:
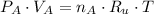
Bulb B (3 L, 4 atm) - Before opening:
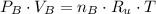
Bulbs A & B (5 L) - After opening:
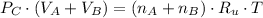
After some algebraic manipulation, a formula for final pressure is derived:
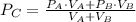
And final pressure is obtained: