The question is incomplete, here is the complete question:
Ethylene is the starting point for a wide array of industrial chemical syntheses. For example, worldwide about 8.0 x 10¹⁰ of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. Natural sources of ethylene are entirely inadequate to meet world demand, so ethane from natural gas is "cracked" in refineries at high temperature in a kinetically complex reaction that produces ethylene gas and hydrogen gas.
Suppose an engineer studying ethane cracking fills a 30.0 L reaction tank with 38.0 atm of ethane gas and raises the temperature to 400°C. He believes Kp = 0.4 at this temperature. Calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture.
Answer: The mass percent of ethylene gas is 9.20 %
Step-by-step explanation:
We are given:
Initial partial pressure of ethane gas = 38.0 atm
The chemical equation for the dehydrogenation of ethane follows:
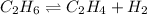
Initial: 38
At eqllm: 38-x x x
The expression of
 for above equation follows:
for above equation follows:
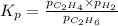
We are given:
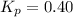
Putting values in above equation, we get:
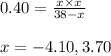
Neglecting the negative value of 'x' because partial pressure cannot be negative
So, equilibrium partial pressure of ethane = 38 - x = 38 - 3.70 = 34.30 atm
Equilibrium partial pressure of ethene = x = 3.70 atm
To calculate the number of moles, we use the equation given by ideal gas which follows:
 ..........(1)
..........(1)
To calculate the number of moles, we use the equation:
 .....(2)
.....(2)
We are given:
![P=34.3atm\\V=30.0L\\R=0.0821\text{ L atm }mol^(-1)K^(-1)\\T=400^oC=[400+273]=673K](https://img.qammunity.org/2021/formulas/chemistry/college/20ol8zerah885hpcu7e4c5jzdzuh7qnegw.png)
Putting values in equation 1, we get:

Molar mass of ethane gas = 30 g/mol
Moles of ethane gas = 18.62 mol
Putting values in equation 2, we get:
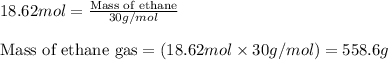
We are given:
![P=3.70atm\\V=30.0L\\R=0.0821\text{ L atm }mol^(-1)K^(-1)\\T=400^oC=[400+273]=673K](https://img.qammunity.org/2021/formulas/chemistry/college/d10k730amqupuzrn8xsbo7h04wdluyz6vm.png)
Putting values in equation 1, we get:

Molar mass of ethylene gas = 28 g/mol
Moles of ethylene gas = 2.01 mol
Putting values in equation 2, we get:

- To calculate the mass percentage of substance in mixture, we use the equation:
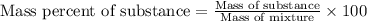
Mass of ethylene = 56.28 g
Mass of mixture = [558.6 + 56.28] g = 641.88 g
Putting values in above equation, we get:
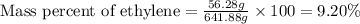
Hence, the mass percent of ethylene gas is 9.20 %