Answer:
The time is 0.713 sec.
Step-by-step explanation:
Given that,
Weight of water = 4 liters
Initial temperature = 128°C
Power = 1400 Watts
Final temperature = 708°C
Weight = 1.1 kg
Specific heat of steel = 0.46 kJ/kg°C
Specific heat of water = 4.18 kJ/kg°C
We need to calculate the heat gained by bucket
Using formula of heat
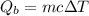
Put the value into the formula
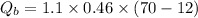
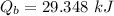
We need to calculate the heat gained by water
Using formula of heat
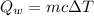
Put the value into the formula
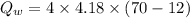
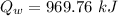
We need to calculate the total heat
Using formula of heat
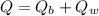
Put the value into the formula
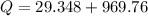
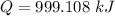
We need to calculate the time
Using formula of time
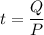
Put the value into the formula
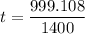
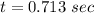
Hence, The time is 0.713 sec.