1) The mass of iron III oxide is 3.92 g and the mass of water is 1.32 g
2) The mass of the Mg is 19.44 g
3) The mass of calcium phosphate is 109 g
What is stoichiometry?
1) The equation is;
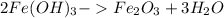
Number of moles of iron III hydroxide
Moles = Mass/Molar mass
= 5.25 g/107 g/mol
= 0.049 moles
2 moles of iron III hydroxide produces 1 mole of iron III oxide
0.049 moles of iron III hydroxide produces 0.049 moles * 1 mole/2 moles
=0.0245 moles
Mass iron III oxide = 0.0245 moles * 160 g/mol
= 3.92 g
2 moles of of iron III hydroxide produces 3 moles of water
0.049 moles of iron III hydroxide produces 0.049 moles * 3 moles/ 2 moles
= 0.0735 moles
Mass of water = 0.0735 moles * 18 g/mol
= 1.32 g
2) Moles of magnesium fluoride = 50 g/62 g/mol
= 0.81 moles

1 mole of Mg produces 1 mole of magnesium fluoride
x moles of Mg produces 0.81 moles of magnesium fluoride
x = 1 * 0.81/1
= 0.81 moles of Mg
Mass of the Mg = 0.81 moles * 24 g/mol
= 19.44 g
3)
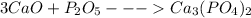
Moles of calcium oxide = 100 g/56 g/mol
= 1.79 moles
Moles of phosphorus pentaoxide = 100 g/284 g/mol
= 0.35 moles
If 3 moles of calcium oxide reacts with 1 mole of phosphorus pentaoxide
1.79 moles of calcium oxide reacts with 1.79 * 1/3
= 0.596 moles
Thus phosphorus pentaoxide is the limiting reactant
1 mole of phosphorus pentaoxide produces 1 mole of calcium phosphate
0.35 moles of phosphorus pentaoxide produces 0.35 moles of calcium phosphate
Mass of calcium phosphate produced = 0.35 moles * 310 g/mol
= 109 g