Step-by-step explanation:
Let us assume that volume of acetic acid added is V ml.
So,
![[CH_(3)COOH] = (0.05 * 100)/(100 + V)](https://img.qammunity.org/2021/formulas/chemistry/college/p60kllp7jytka33ilckxsk2c3f40jk4g6c.png)
and,
![[CH_(3)COONa] = (0.05 * V)/(100 + V)](https://img.qammunity.org/2021/formulas/chemistry/college/vq9u9avl8wq3kn67np51tltm9uqs1trr8g.png)
Expression for the buffer solution is as follows.
pH =
![pK_(a) + log ([CH_(3)COONa])/([CH_(3)COOH])](https://img.qammunity.org/2021/formulas/chemistry/college/cq74yb57a96azauav6fhx9qhfc1m4arp4t.png)
5.1 =
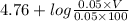
0.34 = log V - 2
log V = 2.34
or, V = 218.77 ml
Now, we will calculate the molarity of the buffer with respect to acetate as follows.
=
![[CH_(3)COO^(-)] + [CH_(3)COOH]](https://img.qammunity.org/2021/formulas/chemistry/college/wmr8xskuzoc7hoccd8nbmjbkcpzpn1dd84.png)
=
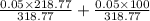
= 0.0499 M
or, = 0.05 M (approx)
Thus, we can conclude that molarity of the resulting buffer with respect to acetate is 0.05 M.