Answer: The equilibrium concentration of NO after it is re-established is 0.55 M
Step-by-step explanation:
For the given chemical equation:
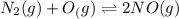
The expression of
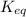 for above equation follows:
for above equation follows:
![K_(eq)=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2021/formulas/chemistry/college/gj7utfulmg2ushbgdgah663wgtw8v03o17.png) .....(1)
.....(1)
We are given:
![[NO]_(eq)=0.400M](https://img.qammunity.org/2021/formulas/chemistry/college/5chvr101pw5y9nlfipf4nhe22tp3jefp0e.png)
![[N_2]_(eq)=0.200M](https://img.qammunity.org/2021/formulas/chemistry/college/t10hkr2roihhfibo3orm06g9ep6okmnpmf.png)
![[O_2]_(eq)=0.200M](https://img.qammunity.org/2021/formulas/chemistry/college/vi0ge3zq8nb54ebr1h4gi5powl3qh1zqxc.png)
Putting values in expression 1, we get:
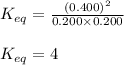
Now, the concentration of NO is added and is made to 0.700 M
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle. This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
The equilibrium will shift in backward direction.
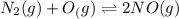
Initial: 0.200 0.200 0.700
At eqllm: 0.200+x 0.200+x 0.700-2x
Putting values in expression 1, we get:
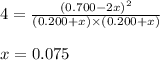
So, equilibrium concentration of NO after it is re-established = (0.700 - 2x) = [0.700 - 2(0.075)] = 0.55 M
Hence, the equilibrium concentration of NO after it is re-established is 0.55 M