Answer: The equilibrium constant for the given reaction is 1.33
Step-by-step explanation:
We are given:
Equilibrium concentration of ammonia = 2 M
Equilibrium concentration of nitrogen gas = 3 M
Equilibrium concentration of hydrogen gas = 1 M
For the given chemical equation:
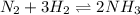
The expression of
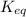 for above equation follows:
for above equation follows:
![K_(eq)=([NH_3]^2)/([N_2][H_3]^3)](https://img.qammunity.org/2021/formulas/chemistry/middle-school/cpettdugrc6fdsiq1p0lakz0t8xw3zp0h6.png)
Putting values in above equation, we get:
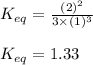
Hence, the equilibrium constant for the given reaction is 1.33