2.61 kilojoules of heat would be liberated by the condensation of 5.00 g of acetone
Step-by-step explanation:
To convert grams into moles
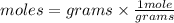
We have 5.00 g acetone
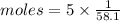
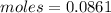
Heat liberated = moles
 heat of vapourization
heat of vapourization
=0.0861 mol x 30.3 kJ/mol
= 2.61 kJ
Therefore, 2.61 kilojoules of heat would be liberated by the condensation of 5.00 g of acetone