339 grams of CLF3 is formed when F2 is in excess and 130 grams of CL2 reacts.
Step-by-step explanation:
The balanced chemical reaction for the formation of ClF3 is given by:
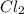 + 3
+ 3
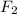 ⇒ 2 Cl
⇒ 2 Cl
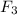
the mass of Cl2 is given 130 grams
From the equation it is found that 2 moles of chloride reacts to form 2 moles of ClF3.
calculating the number of moles of chlorine by the formula:
Number of moles = mass of the substance ÷ atomic mass of one mole of the substance
n = 130 ÷ 35.45
= 3.6671 moles
So, applying stoichiometry
2 moles of Cl2 formed 2 moles of ClF3
3.6671 moles of Cl2 will form x moles of ClF3
2 ÷ 2 = x ÷ 3.6671
x = 3.6671 moles of ClF3
now from the formula of number of moles
weight is calculated as n × mass of the gas
3.6671 × 92.448
= 339.01 grams of ClF3 is formed.