The volume will be 2.25 L.
Step-by-step explanation:
As per Charle's law, the volume of a gaseous system is directly proportional to the temperature provided the system is at constant pressure. Since volume of gas is directly proportional to the temperature of the gas molecules, V∝ T
So, in case there is a change in temperature with constant pressure, then there will be equal change in volume.
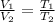
Thus, here
 = 4.50 L ,
= 4.50 L ,
 = 20 °C and
= 20 °C and
 =
=
 = 203 k Pa ,
= 203 k Pa ,
 = 10°C.
= 10°C.
Since, volume and temperature is directly proportional then there will be increase in volume with increase in temperature and vice versa.
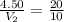
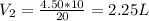
So, the new volume is 2.25 L.