Step-by-step explanation:
According to the law of conservation of mass, mass can neither be created nor it can be destroyed. But it can be simply transformed from one form to another.
Therefore, when
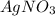 is added to NaCl then the compound formed will have same mass as that of reactants.
is added to NaCl then the compound formed will have same mass as that of reactants.
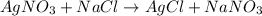
Total mass of reactants is (169.87 + 58.44) g/mol = 228.31 g/mol
Total mass of products is (143.32 + 84.99) g/mol = 228.31 g/mol
Thus, we can conclude that mass of the new mixture will stay the same.