Answer : The fraction of the molecules in the neutral (imidazole) form are, 0.799
Explanation : Given,
pH = 6.6
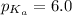
Using Henderson Hesselbach equation :
![pH=pK_a+\log ([Salt])/([Acid])](https://img.qammunity.org/2021/formulas/biology/college/z944fnahhldpjolfrvealc6q9baj5h69q3.png)
![pH=pK_a+\log \frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}](https://img.qammunity.org/2021/formulas/chemistry/college/bib6l7jcuhblhr4k5zoef1jy0xh8q4f8bd.png)
Now put all the given values in this expression, we get:
![6.6=6.0+\log \frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}](https://img.qammunity.org/2021/formulas/chemistry/college/5xaxje1dp0w4hh1dt6r0yd5bte67pd31b9.png)
![\frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}=10^(6.6-6.0)](https://img.qammunity.org/2021/formulas/chemistry/college/pcikldrzp2tan1xvv3an6jutg224xax0k6.png)
![\frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}=10^(0.6)](https://img.qammunity.org/2021/formulas/chemistry/college/v5wrr8tn8yuem4dpqc2azy8z4dpymftn8e.png)
![\frac{[\text{Imidazole}]}{[\text{Imidazolium ion}]}=3.98](https://img.qammunity.org/2021/formulas/chemistry/college/g7s5z5g212zn55r3iom6ukbrxngfsv0rqo.png)
![[\text{Imidazole}]=3.98[\text{Imidazolium ion}]](https://img.qammunity.org/2021/formulas/chemistry/college/1ntr5h68llet2ef84gmfv9kvy87e4idqih.png) ...........(1)
...........(1)
Now we have to determine the fraction of the molecules are in the neutral (imidazole) form.
Fraction of neutral imidazole =
![\frac{[\text{Imidazole}]}{[\text{Imidazole}]+[\text{Imidazolium ion}]}](https://img.qammunity.org/2021/formulas/chemistry/college/p3ewu6dar2dxmqpqmdkd943ywju13595xn.png)
Now put the expression 1 in this expression, we get:
Fraction of neutral imidazole =
![\frac{3.98[\text{Imidazolium ion}]}{3.98[\text{Imidazolium ion}]+[\text{Imidazolium ion}]}](https://img.qammunity.org/2021/formulas/chemistry/college/ab8zua3ktb6kqkrbx96l9906yol48tk0cb.png)
Fraction of neutral imidazole =
![\frac{3.98[\text{Imidazolium ion}]}{4.98[\text{Imidazolium ion}]}](https://img.qammunity.org/2021/formulas/chemistry/college/zpuilxm52cbdsb8e2jbpys1vr9djirzyi8.png)
Fraction of neutral imidazole =
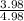
Fraction of neutral imidazole = 0.799
Thus, the fraction of the molecules in the neutral (imidazole) form are, 0.799