Answer:
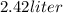
Step-by-step explanation:
The question is incomplete; you need some additional data.
I will assume the missing data from a similar question and keep the final pressure of 703 torr given in your question.
An ideal gas at a pressure of 1.20 atm is contained in a bulb of unknown volume. A stopcock is used to connect this bulb with a previously evacuated bulb that has a volume of 0.720 L as shown here. When the stopcock is opened the gas expands into the empty bulb
If the temperature is held constant during this process and the final pressure is 703 torr , what is the volume of the bulb that was originally filled with gas?
Solution
Since the amount of gas and the temperature remain constant, we may use Boyle's law:
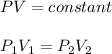
Form the data:
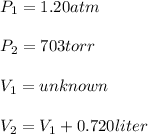
1. Convert 703 torr to atm:
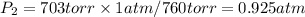
2. Sustitute the data in the equation:
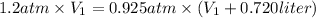
3. Solve:
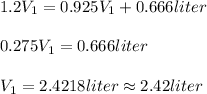
The answer is rounded to 3 significant figures, according to the data.