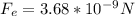Answer:
(a)
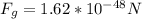
(b)
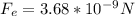
Step-by-step explanation:
(a) We use Newton's law of universal gravitation, in order to calculate the gravitational force between electron and proton:
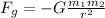
Where G is the Cavendish gravitational constant,
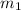 and
and
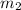 are the masses of the electron and the proton respectively and r is the distance between them:
are the masses of the electron and the proton respectively and r is the distance between them:
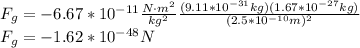
The minus sing indicates that the force is repulsive. Thus, its magnitude is:
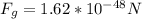
(b) We use Coulomb's law, in order to calculate the electric force between electron and proton, here k is the Coulomb constant and e is the elementary charge:
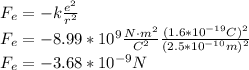
Its magnitude is: