The absolute pressure inside the ball is 1.25atm.
Step-by-step explanation:
Given-
Volume, V = 5..27L
Temperature, T = 27°C
Gauge Pressure, Pg = 0.25 atm
Atmospheric pressure, Patm = 1 atm
Absolute pressure, Pabs = ?
We know,
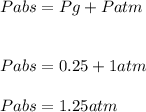
Therefore, absolute pressure inside the ball is 1.25atm.