The question is incomplete, complete question is ;
Allura Red (AR) has a concentration of 21.22 ppm. What is this is micro moles per liter? Report the precise concentration of the undiluted stock solution #1 of AR in micromoles per liter. This is your most concentrated (undiluted) standard solution for which you measured the absorbance. Use 3 significant figures. Molarity (micro mol/L) =
Answer:
The molarity of the solution of allura red is 42.75 micro moles per Liter.
Step-by-step explanation:
The ppm is the amount of solute (in milligrams) present in kilogram of a solvent. It is also known as parts-per million.
To calculate the ppm of oxygen in sea water, we use the equation:
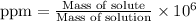
Both the masses are in grams.
We are given:
The ppm concentration of allura red = 21.22 ppm
This means that 21.22 mg of allura red was present 1 kg of solution.
Mass of Allura red = 21.22 mg =
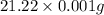
1 mg = 0.001 g
Mass of solution = 1 kg = 1000 g
Density of the solution = Density of water = d = 1.00 g/mL
( since solution has very small amount of solute)
Volume of the solution :
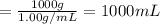
1000 mL = 1 L
Volume of the solution, V = 1 L
Moles of Allura red =
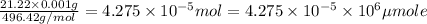
Molarity of the solution ;
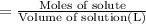
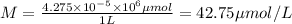
The molarity of the solution of allura red is 42.75 micro moles per Liter.