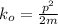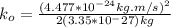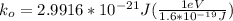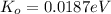Answer:
0.0187 eV
Step-by-step explanation:
Given that:
diameter of the hydrogen gas (λ) = 1.48 ×10⁻¹⁰ m'
mass of the hydrogen gas = 3.35 ×10⁻²⁷ kg
We need to determine the momentum first before calculating the kinetic energy.
So momentum of the hydrogen gas molecule is written as;
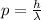
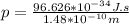
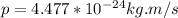
NOW, the kinetic energy of the hydrogen gas molecule is calculated as follows by using the formula: