- The balanced equation with the missing coefficient is given as
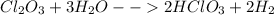
- This type of reaction is called as Double Displacement reaction.
Step-by-step explanation:
- The given equation is Cl2O3 + H20 ---> HCIO3 + H2. For the reaction to be balanced, the number of atoms of each element must be equal on both sides of the reaction arrow.
- On the left side, we have
Cl: 2
O : 3 + 1 = 4
H: 2
H : 1 + 2 = 3
Cl: 1
O : 3
- You will notice that there is a shortage of Chlorine and Oxygen atoms on the right side and the Hydrogen atom is not correctly balanced. To make it a balanced ratio, add 3 to the H2O on the left side, 2 to the HClO3, and 2 to the H2.
Now the balanced equation becomes,
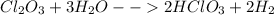
- This type of reaction is called a Double Displacement reaction in which an acid and a base react in aqueous solution to form a salt and (usually liquid) water.