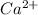Answer: The symbol of the ion formed is
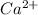
Step-by-step explanation:
An ion is formed when a neutral atom looses or gains electrons.
- When an atom looses electrons, it results in the formation of positive ion known as cation.
- When an atom gains electrons, it results in the formation of negative ion known as anion.
Electronic configuration is defined as the representation of electrons around the nucleus of an atom.
Number of electrons in an atom is determined by the atomic number of that atom.
The element present in Group 2-A and in period 4 is Calcium (Ca)
Electronic configuration of Ca atom:
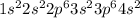
This atom will loose 2 electrons to attain stable electronic configuration similar to Argon element (noble gas)
The electronic configration of
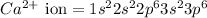
Hence, the symbol of the ion formed is