Answer:
160.4K
Step-by-step explanation:
Given parameters:
Initial volume of gas = 2.9L
Initial pressure of gas = 5atm
Initial temperature = 50°C
Final volume = 2.4L
Final pressure of gas= 3atm
Unknown:
Final temperature = ?
Solution:
To solve this problem, we have to apply the combined gas laws.
Mathematically, the law is expressed as;
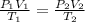
where P₁ is the initial pressure
V₁ is the initial volume
T₁ is the initial temperature
P₂ is the final pressure
V₂ is the final volume
T₂ is the final temperature
Since T₂ is the unknown, we must solve for it.
convert T₁ from °C to K;
T₁ = 273 + 50 = 323K
Now insert the parameters;
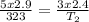
 = 160.4K
= 160.4K