The given question is incomplete. The complete question is as follows.
A chlorine atom is adsorbed on a small patch of surface (see sketch at right). This patch is known to contain 81 possible adsorption sites. The atom has enough energy to move from site to site, so it could be on any one of them. Suppose a Br atom also becomes adsorbed onto the surface. Calculate the change in entropy. Round your answer to significant digits, and be sure it has the correct unit symbol.
Step-by-step explanation:
The change in entropy will be calculated by using the following formula.
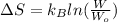
Initially, it is given tha Cl atom could be adsorbed on any of the 81 sites. When Br is added then there will be 80 possible sites when the Br can be adsorbed. This means that total possible sites are as follows.
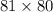
= 6480
This shows that there are 6480 microstates which are accessible to the system.
So, change in entropy will be calculated using the Boltzmann constant as follows.
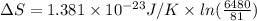
=
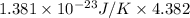
=
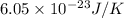
or, =
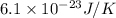 (approx)
(approx)
Thus, we can conclude that the change in entropy is
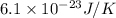 .
.