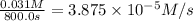Answer:
a) 0.000112 M/s is the average reaction rate between 0.0 seconds and 1500.0 seconds.
b) 0.00011 M/s is the average reaction rate between 200.0 seconds and 1200.0 seconds.
c) Instantaneous rate of the reaction at t=800 s :
Instantaneous rate :
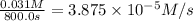
Step-by-step explanation:
Average rate of the reaction is given as;
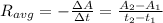
a.) The average reaction rate between 0.0 s and 1500.0 s:
At 0.0 seconds the concentration was =
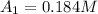
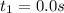
At 1500.0 seconds the concentration was =
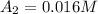
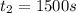
![R_{avg]=-(0.016 M-0.184 M)/(1500.0 s-0.0 s)=0.000112 M/s](https://img.qammunity.org/2021/formulas/chemistry/college/9kq2jwybf5lhokguq1g9v06am889v7jc90.png)
0.000112 M/s is the average reaction rate between 0.0 seconds and 1500.0 seconds.
b.) The average reaction rate between 200.0 s and 1200.0 s:
At 0.0 seconds the concentration was =
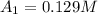
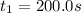
At 1500.0 seconds the concentration was =
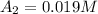
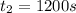
![R_{avg]=-(0.019 M-0.129M)/(1200.0s-200.0s)=0.00011 M/s](https://img.qammunity.org/2021/formulas/chemistry/college/cn51ojj2gr9wn2ka2bs02gubcc5fuz9dxs.png)
0.00011 M/s is the average reaction rate between 200.0 seconds and 1200.0 seconds.
c.) Instantaneous rate of the reaction at t=800 s :
At 800 seconds the concentration was =
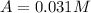
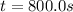
Instantaneous rate :