Answer:
The balanced chemical equation for this process:
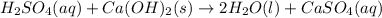
$194.51 is the cost that the firm will incur from this use of slaked lime.
Step-by-step explanation:
The balanced chemical equation for this process
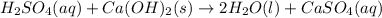
Moles of sulfuric acid = n
Volume of sulfuric acid disposed = V = 1200 gallons = 3.785 × 1200 L = 4,542 L
1 gallon = 3.785 Liter
Morality of the sulfuric acid = M = 1.05 m
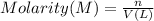
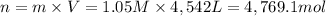
According to reaction, 1 mol of sulfuric acid reacts with 1 mole of calcium hydroxide.Then 4,769.1 moles of sulfuric acid will recat with ;
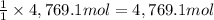 of calcium hydroxide
of calcium hydroxide
Mass of 4,769.1 moles of calcium hydroxide:
4,769.1 mol 74 g/mol = 352,913.4 g
=
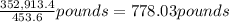
(1 pound = 453.6 grams)
Cost of 1 pound of slaked lime = $0.25
Cost of 778.03 pounds of slaked lime = $0.25 × 778.03 = $194.51
$194.51 is the cost that the firm will incur from this use of slaked lime.