Answer:
67.3 s
Step-by-step explanation:
The equation for the reaction can be represented as:
N₂O₅ ⇄ NO₂ + O₂
Rate (k) =

Rate law for first order is expressed as:
In [A] = -kt + In [A]₀
Given that:
[A] = Final Concentration = 1.85 M
[A]₀ = Initial Concentration = 2.75 M
time-taken = ???
substituting our given data; we have:
In[1.85] = -[5.89 × 10⁻³](t) + In [2.75]
t =
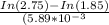
t =
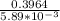
t = 67.3 s