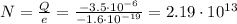Answer:
Step-by-step explanation:
The electron is a fundamental particle, with a charge of
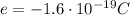
which is also known as fundamental charge.
For an object having N excess electrons, the total charge on the object is
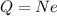 (1)
(1)
where e is the charge of the electron.
For the object in this problem, its charge is
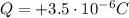
This can be obtained by removing a negative charge equal to
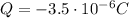
Substituting into (1) and solving for N, we can find the number of electrons: